Dive with Visium HD: Uncover Spatial Cell Communication at Single-Cell Clarity
In the ever-changing world of biological research, understanding how cells communicate within their spatial context is crucial. The 10X Visium HD technology has become a game-changer, allowing scientists to study cell interactions with amazing detail and clarity. In this post, we explore the capabilities of the Visium HD dataset to dissect cell-cell communication in a spatial context at single-cell precision.
We use the colorectal cancer dataset from 10X Genomics, which you can download here, and analyze the data using our tool, OmnibusX. Our analysis is broken down into three key steps:
- Cell type annotation.
- Analyzing cell type composition in tissue context.
- Analyzing cell-cell Interactions in the spatial context.
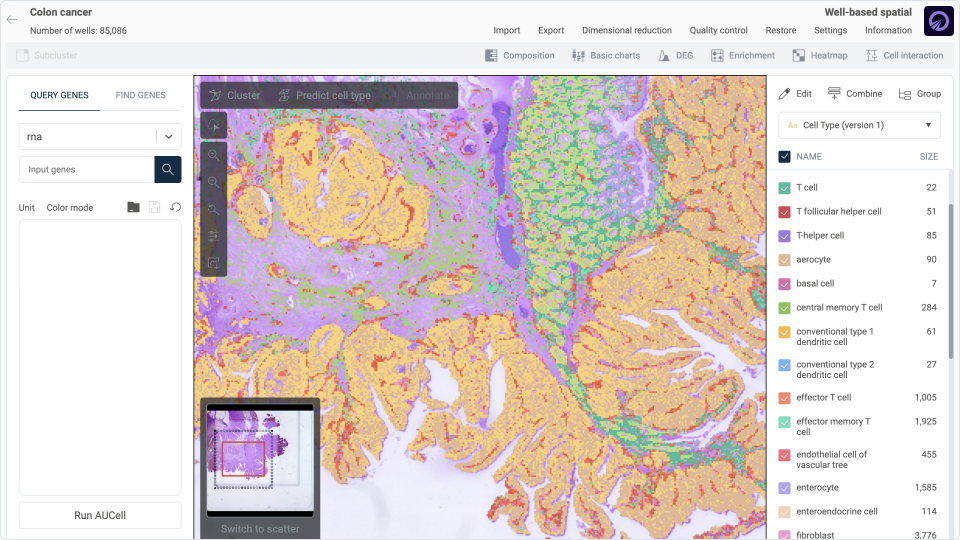
Step 1: Cell type annotation
In this initial step, cell type labels are predicted automatically using our comprehensive single-cell database. Our detailed methodology is outlined here. Through this process, we assign labels to each cell at two hierarchical levels: major cell types and their respective subtypes. For the simplicity of this discussion, we will focus on the major cell types. The below summary of cell type labels demonstrate consistency with the tissue structure observed in the corresponding image.
Step 2: Analyzing cell type composition in tissue context
In the tissue image, we can see an intact normal tissue region, which is easily recognized by its normal structure: well-organized, uniformly spaced glands. These glands are tubular with a smooth, rounded appearance and are lined by a single layer of columnar epithelial cells. The glands are similar in size and shape, maintaining a consistent pattern. The mucosa in normal regions appears intact with clear layers of epithelium, lamina propria, and muscularis mucosae. The epithelium is neatly aligned with goblet cells.
In contrast, the cancerous region shows a distinct morphology: the glands vary in shape and size, and some appear distorted and irregular. There are areas where the glandular architecture is highly disorganized, with irregularly shaped and crowded glands. The connective tissue stroma appears desmoplastic, meaning it has a dense fibrous reaction, a common response in invasive cancers. This stroma is interspersed between the neoplastic glands and contributes to the irregular tissue architecture.
Correlating these morphological details with expression data, we found a strong agreement.
1. Goblet and Enterocyte distribution
The distribution of goblet cells and enterocytes within the normal tissue regions aligns remarkably well with their respective tissue image morphologies. Predominantly found in these areas, both cell types maintain a well-organized structural arrangement. Goblet cells, marked in blue, are interspersed among the orange-labeled enterocytes, mirroring the healthy, intact mucosal lining observed in the histological image. This spatial arrangement underscores the functional integrity of the epithelium, which is crucial for maintaining barrier protection and nutrient absorption in the gastrointestinal tract.
2. Transit amplifying cells and Intestinal crypt stem cells distribution
In the normal tissue regions, the transit amplifying cells and intestinal crypt stem cells, depicted in teal and red respectively, constitute a modest fraction of the cellular composition, accurately reflecting their limited but crucial roles in maintaining intestinal homeostasis as seen in the image morphology. However, in the cancerous regions, these cell types show a dramatic increase in number and density. This significant growth within the tumor areas suggests that these cells might be the origin of cancer cells, transforming from progenitor cells into the main contributors to the tumor mass. The clear visualization and distinct separation of these cell populations in the two regions indicate their pivotal roles in both normal cellular turnover and cancer progression.
3. Fibroblast and pericyte distribution
In the cancerous regions, the stromal compartment, particularly marked by the presence of fibroblasts and pericytes, shows a dense infiltration that is clearly visible in the image. Fibroblasts, which are abundant throughout the tissue, and pericytes, which are closely associated with blood vessels, together contribute to a strong stromal reaction. This extensive growth aligns with the features seen in aggressive tumor growth, where fibroblast activation plays a crucial role in remodeling the extracellular matrix and creating an environment that supports the growth and spread of mutated cancer cells. The presence of these stromal cells within the tumor matrix not only highlights their important role in cancer progression but also suggests their potential as targets for therapy to disrupt the tumor-supportive environment.
4. Immune cells distribution
In this tissue sample, the immune cell population is predominantly composed of plasma cells, macrophages, and T cells. Plasma cells are primarily localized in the normal regions of the gastrointestinal mucosa, which typically harbors numerous plasma cells under healthy conditions. This distribution aligns with their conventional role in mucosal immunity, where they produce antibodies to protect against pathogens. Intriguingly, plasma cells also manifest prominently within the cancerous regions, forming distinct, dense clusters. This unusual concentration suggests an active immune response, potentially indicative of the body's effort to combat the malignancy.
Macrophages are evident within both the normal and cancerous regions of the tissue. In the normal tissue, these cells are sparsely distributed, performing their routine roles in immune surveillance and tissue homeostasis. However, the scenario shifts dramatically within the cancerous regions, where macrophages are densely populated and actively infiltrate along the blood vessels. Such infiltration is often associated with both tumor-fighting and, paradoxically, tumor-promoting activities, depending on the signals received from the cancer cells.
Within the tissue sample, T cells exhibit three distinct populations with varying tissue distributions, highlighting their roles in immune surveillance and response. Effector memory T cells are sparsely distributed throughout the normal regions, indicating their role in maintaining immune memory without immediate proximity to the tumor site. In contrast, these cells densely populate streams away from the cancerous regions, potentially patrolling and preparing for activation upon tumor interaction. Effector T cells, however, are notably concentrated in distinct, dense spots within the cancerous regions, suggesting active engagement in immune responses against tumor cells. Central memory T cells are also visible within the cancerous areas, where they, along with other immune cell types, form lymphoid-like structures. This arrangement is crucial for sustaining long-term immune responses and for orchestrating complex interactions with both the tumor cells and other components of the immune system, thereby contributing to a structured defense mechanism within the tumor microenvironment.
5. Cell type composition in lymphoid-like structure
In our detailed examination of a lymphoid-like structure within the cancerous region, the cell type composition closely mirrors the elements typically found in tertiary lymphoid structures, which are known to emerge in chronic inflammatory conditions, including cancer. The structure predominantly features a variety of immune cells such as B cells, CD4 T cells, dendritic cells, macrophages, and T follicular helper cells. These cells are intricately arranged and appear to interact dynamically, forming a localized immune response hub that could potentially influence tumor behavior. Surrounding these immune cells, stromal components including fibroblasts, myofibroblasts, pericytes, and endothelial cells create a supportive scaffold that not only maintains the structural integrity of the lymphoid-like structure but also facilitates cellular interactions and migration. This complex cellular arrangement suggests an adaptive immune microenvironment within the tumor, which might play crucial roles in both promoting and inhibiting tumor progression.
Step 3: Analyzing cell-cell Interactions in the spatial context
In this step, we explore the dynamic interactions between cells within the tissue microenvironment. We obtain interaction information from CellphoneDB and biological processes from the Gene Ontology. Our initial step involves identifying specific cellular locales that express pairs of interacting molecules as defined by CellphoneDB. Subsequently, we map these interactions to relevant biological processes, facilitating a targeted retrieval of functional pathways involved. Following identification, we conduct enrichment analysis to determine which of these biological processes are actively influencing the tissue context. This analysis helps in highlighting the most prominent pathways that may be driving pathological or therapeutic responses within the tissue. All of these functions are integrated into the OmnibusX application. You can download the application here to save time processing your data.
1. Specific interaction at lymphoid-like structure
In the lymphoid-like structures identified within the tissue, our focused examination of cell-cell interactions has revealed significant and unique interactions exclusive to this region. Specifically, we observed two unique interactions involving the ligand-receptor pairs CCL19-CCR7 and CCL21-CCR7. These interactions are integral to the immune response functions within this microenvironment.
The interaction between CCL19 and its receptor CCR7 is associated with three enriched biological processes:
- GO_0002606: positive regulation of dendritic cell antigen processing and presentation
- GO_0002408: myeloid dendritic cell chemotaxis
- GO_0097029: mature conventional dendritic cell differentiation
Similarly, the interaction between CCL21 and CCR7 facilitates processes related to two biological processes: the chemokine (C-C motif) ligand 21 signaling pathway (GO_0038116) and again, the positive regulation of dendritic cell antigen processing and presentation (GO_0002606).
- GO_0038116: chemokine (C-C motif) ligand 21 signaling pathway
- GO_0002606: positive regulation of dendritic cell antigen processing and presentation
These findings highlight the specialized roles these interactions play in orchestrating immune activities within the lymphoid-like structures, significantly influencing dendritic cell function and migration, which are crucial for initiating and regulating immune responses against the tumor.
2. Interactions related to metastasis process
In the analysis of tumor metastasis processes, our focus is drawn to specific interactions occurring at the outermost layer of the tumor mass, identified as critical zones for potential spread.
One such interaction involves PLAU ligand and its receptor PLAUR, which prominently occurs in this peripheral region. This interaction is associated with three enriched biological processes:
- GO_0010755: regulation of plasminogen activation
- GO_0038195: urokinase plasminogen activator signaling pathway
- GO_0051917: regulation of fibrinolysis
These processes facilitate the breakdown of extracellular matrices, potentially enabling tumor cells to invade neighboring tissues and enter the bloodstream.
Additionally, the interaction between EFNA1 ligand and EPHA2 receptor, also localized at the tumor’s edge, links to critical pathways that influence the tumor environment:
- GO_0043535: regulation of blood vessel endothelial cell migration
- GO_0014028: notochord formation
This suggests a role in promoting the angiogenesis necessary for tumor growth and metastasis, by influencing endothelial dynamics and possibly contributing to the structural organization within the tumor. Together, these interactions indicate a highly orchestrated network at the tumor margin, guiding both the invasive potential and the angiogenic processes that are pivotal in the metastatic cascade.
Conclusion
This analysis offers just a preliminary glimpse into the complex cellular dynamics and interactions within the tumor microenvironment. The interactions we've explored between various ligands and receptors, particularly at the critical edges of the tumor mass, highlight the sophisticated regulatory networks that may contribute to tumor growth and metastasis. However, this is merely the tip of the iceberg. There are many more layers of information and cellular interactions waiting to be uncovered in these samples.
At OmnibusX, we are committed to simplifying and streamlining this complex process to facilitate and accelerate the discovery of new cellular insights. Your engagement and curiosity drive the scientific community forward, and we look forward to sharing more discoveries with you in the future. Thank you for reading, and remember that your efforts are crucial in this uncharted territory of scientific exploration.
We invite experts in the field to join us in delving deeper into these findings, to expand our understanding and potentially uncover new therapeutic targets. Your expertise could be crucial in unraveling the intricate biological processes that drive cancer progression and in translating this knowledge into meaningful clinical outcomes.
